The latest edition of Oppenheimer & Co. Inc.’s Quarterly Biopharma M&A and Strategic Collaboration Insights Report is now available upon request.
Key Takeaways:
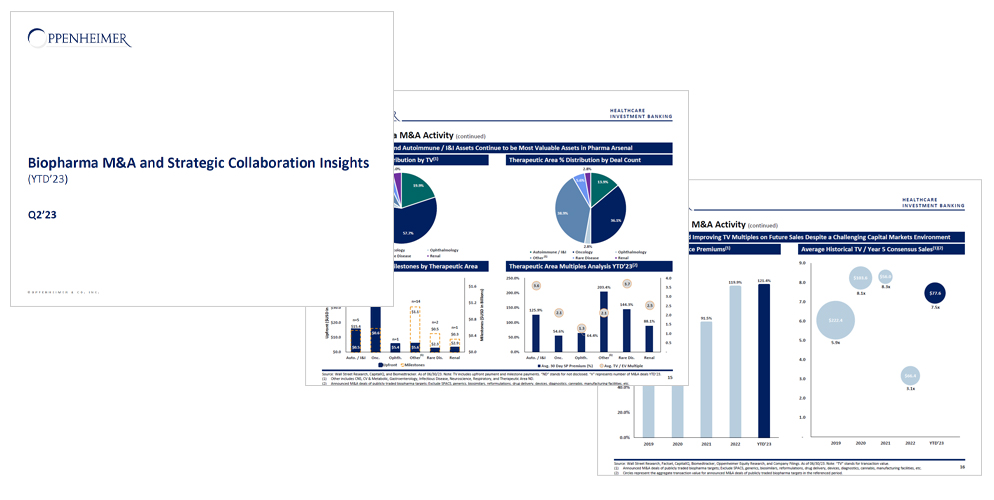
YTD’23 M&A Activity Has Already Cleared FY’21 Levels and Is on Track to Surpass FY’22
-
2023 Biopharma M&A started off blazing hot with the $42.8B acquisition of Seagen and several notable $1B+ acquisitions including Prometheus Biosciences, IVERIC bio, Chinook Therapeutics and Provention Bio
- Additionally, there has been considerable acquisitions of assets in FY’23, including Bausch + Lomb’s purchase of Novartis’ XIRDA for $2.5B and Amphastar Pharmaceuticals’ acquisition of Baqsimi for $1.1B (both including milestones).
- At a high level, Q2’23 activity was comparable to Q1’23 in terms of aggregate transaction value, deal counts, and asking prices
- Premiums for publicly traded targets in Q2’23 remained in line with Q1’23 levels (~120%): up from FY’21 and comparable to FY’22 levels (92% and 120% respectively)
- Commercial and clinical stage companies make up the vast majority of YTD’23 M&A activity (~63% and ~34% of total TV): biologics (ADCs and mAbs) representing a new favorite among investors, while there is still significant interest in small molecule companies despite continued pressure exerted by IRA legislation
-
In terms of strategic collaboration activity, Q2’23 was in line with Q1’23; however, upfront cash payments were down slightly while milestone payments increased: likely a reflection of the continued challenges in the equity market
-
There was a substantial increase in strategic collaboration deals for discovery / platform stage companies during Q2’23
-
Small molecules, ADCs, and cell & gene therapy focused companies remain the hottest targets for partnerships on a treatment modality basis
-
Oncology and CNS companies represent the most active therapeutic areas for strategic collaborations
-
Recent earnings calls with Big Pharma support a continued appetite for M&A that will be fueled by IRA implications and a need for top line growth to drive shareholder value
Deal Catalysts
-
Excess cash reserves at big pharma and large biotech and the need for top line growth
-
Steep and fast‐approaching patent cliff for big pharma
-
Mega‐blockbusters set to lose exclusivity over the next six years represent the biggest threat to commercial drug sales in decades
-
Three drugs facing near‐term loss of exclusivity have generated $50.5B in combined LTM global revenues alone: Merck’s Keytruda ($21.9B), AbbVie’s, Humira ($20.0B), and BMS’ Opdivo ($8.5B)
-
A challenging IPO market underpinned by low biotech valuations
-
Profound clinical data and positive newsflow
-
Declining COVID‐19 vaccine revenue
Deal Headwinds
-
Ambiguous macroeconomic outlook coupled with rising costs/inflation concerns
-
Increased FTC scrutiny (e.g. FTC issued Opinion and Order for Amgen / Horizon and Illumina / Grail)
-
Fragile supply chains and escalating costs of talent / human capital
-
Decreasing risk appetite to take on early‐stage programs whose funding requirements will eat into profits
-
Bottom line P&L impacts from the new drug price negotiation legislation beginning in 2026 signed in to law through the IRA
Please reach out to Michael Margolis, R.Ph. ([email protected]), Daniel Parisotto, Ph.D ([email protected]), or Robert Lewis ([email protected]) directly to request a copy.

Michael A. Margolis, R.Ph.
Title:Senior Managing Director, Co-Head of Healthcare, Head of Healthcare Life Sciences
DISCLOSURES
This notice is provided for informational purposes only, and is not intended as a recommendation or an offer or solicitation for the purchase or sale of any security or financial instrument. Nothing contained herein shall constitute an offer or solicitation to buy or sell any securities discussed herein in any jurisdiction where such offer or solicitation would be prohibited.
This notice may contain statistical data cited from third-party sources believed to be reliable, but Oppenheimer & Co. Inc. does not represent that any such third-party statistical information is accurate or complete, and it should not be relied upon as such. All market prices, data and other information are not warranted as to completeness or accuracy and are subject to change without notice.
2023 Oppenheimer & Co. Inc. Transacts Business on all Principal Exchanges and Member SIPC 5803381.1

