The Race for a Vaccine Explained
- September 28, 2020
An overview of the current status of Covid-19 vaccine research, possible timelines for approvals and the obstacles that could delay the process.
In the race for a Covid-19 vaccine, there is both extreme urgency and stiff competition. Under normal circumstances, a vaccine can take up to 10 years to get approved but the scope of this outbreak has fueled efforts to accelerate that timeline dramatically. While many obstacles remain and no timeline is certain, several vaccine candidates currently under development may soon be available for emergency use.
5 Stages of Vaccine Development
Step-by-step approval process
| Phase | No. of Candidates | Overview |
|---|---|---|
| Pre-Clinical | 92 | Research is first conducted on animals to determine whether the vaccine will produce immunity and to determine how quickly the drug can be digested and excreted from the body. |
| Phase 1 | 27 | Vaccine is administered to a small group of humans to assess the safety and potential responses among subjects. |
| Phase 2 | 9 | Vaccine is administered to larger groups (hundreds) of people to learn more about safety and biological response. Dosage size is also more widely tested during this stage of the process. |
| Phase 3 | 5 | Vaccine is administered to larger group (thousands) of people including a control group that receives a placebo. The stage is used to confirm safety, identify rare side effects and measure the drug’s effectiveness. |
| Approved | 0 | The developer of the vaccine must submit results to government authorities, scientists and advisory committees, which review results to verify claims of safety and effectiveness. The drug is then eligible for broader production and distribution. |
Source: New York Times, World Health Organization, National Institute of Allergy and Infectious Diseases, National Center for Biotechnology Information, New England Journal of Medicine
Of course, no vaccine will ever be 100% effective because viruses inherently have a propensity to mutate over time, according to the World Health Organization. Preliminary research reports show that Covid-19 may be similar to the seasonal flu in that new strains may emerge every year, requiring slight modifications to an existing vaccine. Over time, diseases tend to mutate into less aggressive strains as a virus’ main goal is to stay alive longer in its host. If the host dies too quickly, then the virus itself will cease to exist. Some experts believe this could explain why the fatality rate has decreased despite the rising number of cases globally.
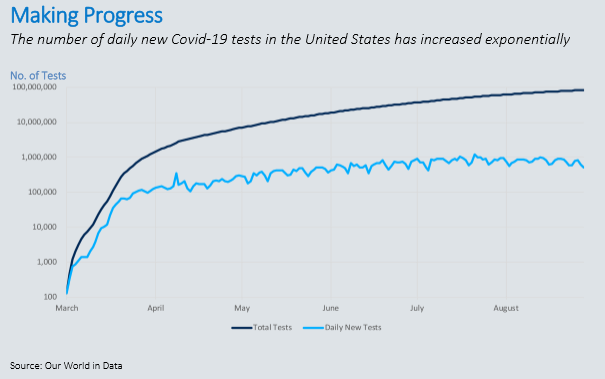
At the start of the outbreak, Covid-19 tests were reserved for patients in critical condition who required immediate medical attention. Given that the virus was previously unidentified and highly contagious, the global medical supply chain wasn’t prepared to provide adequate supplies, equipment and testing. Today, the United States is conducting hundreds of thousands of tests daily, many with a turnaround time of a few hours to a few days. That illustrates the progress we’ve made from the limited testing and week-long response times we saw early on.

Hurdles to Clear
As several vaccine candidates approach the final stage of development, there are four potential obstacles:
1. Once approved, there will be overwhelming demand but limited supply.
2. Current supply chains need to be expanded to distribute the vaccine in a timely manner.
3. Temperature controls are required to stabilize the vaccine and maintain its efficacy.
4. The first drug makers to market with a vaccine may reap financial benefits through licensing rights, intellectual property and branding. But the vaccine may not have been sufficiently tested beyond FDA requirements. Meanwhile, vaccines offered later may gain exposure to a larger pool of patients in clinical trials.
A Faster Track
While the typical vaccine research and development process can take up to 10 years, the critical nature of the pandemic has led to attempts to shorten this timeline. Global research efforts began in early January 2020 with the first vaccine trials on humans beginning in March, a process that typically takes three to five years. This acceleration can be attributed to four factors:
- Fast-track regulatory approvals. Vaccine candidates have received emergency regulatory approval to skip years of animal trials in an effort to speed up the process. Some organizations have combined research phases, conducting phase 1 and phase 2 trials simultaneously.
- Innovative vaccine methodology. Advances in bioengineering technology have sped up the time required for genome sequencing and testing. Messenger RNA (mRNA), a new vaccine methodology, gives the body instructions on how to produce disease-specific antibodies on its own.
- Public and private sector support. Global support from the public and private sector has fueled funding for research efforts. The United States launched Operation Warp Speed, which is aimed at accelerating the development, manufacturing and distribution of Covid-19 vaccines, therapeutics and diagnostics. $10 billion was allocated to this program through various funding programs including the Cares Act.
- Scientists’ familiarity with coronaviruses. Prior knowledge of the coronavirus family has aided scientists in their research efforts. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), more commonly referred to as Covid-19, and SARS—the strain from the 2003 outbreak—are roughly 80% identical in nature, according to several studies. By using existing research, scientists have been able to accelerate discovery related to a potential vaccine.
A Second Shutdown?
Despite the rising global case count, the seven-day daily death moving average has been declining since its peak in April. This encouraging trend can be attributed to the improved availability of tests and more available data to guide patient treatment and care. As the world braces for a highly uncertain winter, it will be important to track how spending more time indoors and the return of flu season might impact virus transmission.
Fortunately, the global response to Covid-19 has been unprecedented, with accelerated research timelines and emergency approval from regulatory agencies to address the virus. With hundreds of candidates currently undergoing trials with several in the later stages, there is hope but by no means certainty, that a vaccine will be approved and widely available in the next six to nine months. Historically, there have been many obstacles that stood in the way of vaccine development, which can slow down the approval process. The need for the global supply chain to distribute a vaccine at scale has forced public and private sectors to come together to best address this issue.
Still, the willingness of the people to receive a vaccination could be a major hurdle. Media reports are highlighting recent polls showing that confidence in the safety of a fast-tracked vaccine is waning. While a vaccine and a potential resurgence of the virus will dominate the headlines, we believe another round of broad-based lockdown measures is unlikely given the deep economic damage they have caused. Ultimately, urgency, competition and collaboration should benefit vaccine research and development.
Disclosure
This material is intended for informational purposes only. The information and statistical data contained herein have been obtained from sources we believe to be reliable. The opinions expressed are those of Oppenheimer Asset Management Inc. (“OAM”) and its affiliates are subject to change without notice. No part of this material may be reproduced in any manner without the written permission of OAM or any of its affiliates. Any securities discussed should not be construed as a recommendation to buy or sell and there is no guarantee that these securities will be held for a client’s account nor should it be assumed that they were or will be profitable. Securities referenced herein are used as proxies or illustrations of broader market or sector principles. Past performance does not guarantee future comparable results. All securities investing entails some risk including of loss of principal.
Oppenheimer Asset Management is the name under which Oppenheimer Asset Management Inc. (OAM) does business. OAM is a registered investment adviser. A strategy's performance may be affected by general economic and market conditions, such as interest rates, inflation rates, economic uncertainty, changes in laws, and national and international political circumstances. The information provided herein should not be construed as a recommendation to buy, sell, or hold any particular security. Opinions expressed herein are current as of the date appearing in this material.
OAM is an indirect wholly owned subsidiary of Oppenheimer Holdings Inc. which also indirectly wholly owns Oppenheimer & Co. Inc. (“Oppenheimer”), a registered broker/dealer and investment adviser. Securities are offered through Oppenheimer and will not be insured by the FDIC or other similar deposit insurance, will not be deposits or other obligations of Oppenheimer or guaranteed by any bank or other financial institution, will not be endorsed or guaranteed by Oppenheimer and will be subject to investment risks, including the possible loss of principle invested. This commentary may contain forward looking statements or projections. These statements and projections relate to future events or future performance.
Forward-looking statements and projections are based on the opinions and estimates of Oppenheimer as of the date of this presentation, and are subject to a variety of risks and uncertainties and other factors, such as economic, political, and public health, that could cause actual events or results to differ materially from those anticipated in the forward-looking statements and projections. 3248273.1